Absci R&D Day 2023
Oct 04, 2023
Absci unveils its internal drug pipeline, recent technical and business milestones, and a peek into the future of AI drug creation.
On October 4, 2023, Absci hosted its inaugural R&D Day, welcoming investors, research analysts, current and potential partners, and media to hear highlights of its progress, technologies, and future plans in AI drug creation. Key takeaways:
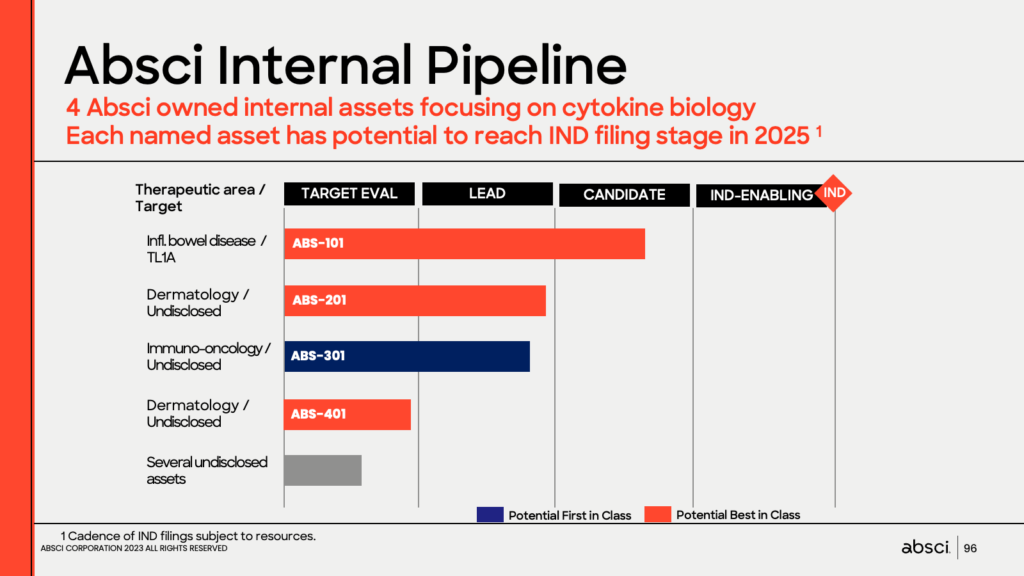
- Absci revealed its internal drug discovery pipeline, including four wholly-owned assets focused on cytokine biology each with the potential to reach the IND filing stage in 2025, as well as several undisclosed assets.
- Pipeline candidate ABS-101, which targets TL1A, demonstrates the potential for a best-in-class profile in a ~$24 billion market to treat inflammatory bowel disease and many other inflammatory and fibrotic diseases.
- Absci reported the discovery of a potentially novel mechanism of tumor immune evasion using reverse immunology in the development of ABS-301, with broad first-in-class potential in immuno-oncology.
- Absci shared progress on scaling and integrating AI drug creation operations, including estimated improvements of greater than 15% in R&D workflow efficiencies, and its business strategy for developing a diversified portfolio of internal and partnered programs.
Watch the Absci R&D Day webcast
Special guest Sandi Peterson, Lead Independent Director at Microsoft and Operating Partner at Clayton Dubilier & Rice, provided opening remarks. At Johnson & Johnson, Peterson helped reinvent a legacy company as a cutting-edge science and technology firm. Peterson said that most people underestimate how long it takes to commercialize these kinds of technologies, but her sense is that Absci is on the cusp of something quite different: “We have real targets, we have a real pipeline, we have a lot of exciting things going on here.” She added that Absci is combining talent and technology in a way that promises to “completely change the timelines” of what’s possible in drug discovery.
Special guest Jonathan Cohen, VP of Applied Research at NVIDIA, shared his perspective on generative AI for life sciences. He provided some technical background on Large Language Models (LLMS) underpinning generative AI, the zero-shot approach that epitomizes foundation models and Absci’s de novo approach, and how NVIDIA thinks about achieving “million-X speedups” to fully realize the promise of AI and scientific computing in life sciences.
From drug discovery to drug creation
Sean McClain, Absci’s Founder & CEO, reiterated the need to transform the drug discovery paradigm. McClain described the current challenges in the field, including the time-consuming process from discovery to IND, the low (~5%) success rate from discovery to launch, and processes that result in candidates with suboptimal attributes. He asked: “What if the next transformative drug was not discovered but created with a click of a button?”
McClain introduced Absci’s approach to drug discovery: leveraging scalable biological data and artificial intelligence (AI). He described how Absci’s E. coli SoluPro™ cell lines and ACE Assay™ technology generate massive, high-quality training data for Absci’s drug creation platform. This integrated approach aims to speed the iteration and improvement of AI models, reduce preclinical development timelines, and increase the clinical probability of success.
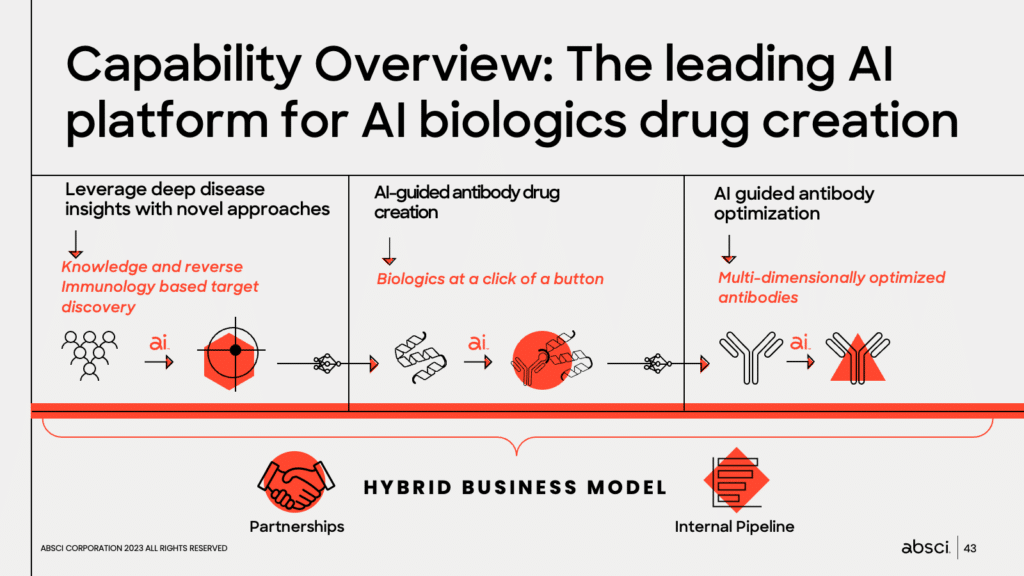
Chief Innovation Officer Andreas Busch provided an overview of Absci’s capabilities. A pharma veteran who has brought several blockbusters from bench to approval, Dr. Busch said that Absci aims to do nothing less than build the leading AI platform for biologics drug creation.
More about Absci’s drug creation platform
Introducing Absci’s Internal Pipeline
Christian Stegmann, SVP for Drug Creation, unveiled Absci’s internal pipeline. Absci is currently developing four wholly-owned assets, each focusing on cytokine biology, and each with the potential to reach the IND filing stage in 2025. Stegmann provided an overview of each asset, including ABS-101, a drug candidate targeting TL1A antagonism for the treatment of inflammatory bowel disease. He highlighted ABS-101’s potential to become a blockbuster in inflammatory and fibrotic diseases due to its potential for a best-in-class profile compared to existing treatments.
Stegmann explained how Absci’s reverse immunology technology elucidated a potentially novel mechanism of tumor immune evasion in conjunction with the development of ABS-301, which has broad first-in-class potential in immuno-oncology. Stegmann said that several other undisclosed assets are in the target evaluation stage.
Revolutionizing biologics drug discovery with AI
Amaro Taylor-Weiner, Absci’s Chief AI Officer, described how Absci uses generative AI to develop novel antibodies. He explained that the company’s approach combines deep disease insights with novel approaches such as reverse immunology and de novo antibody discovery to create biologics at the click of a button. He also highlighted the ability of Absci’s AI to optimize antibodies multi-dimensionally, leading to enhanced attributes of the resultant biologics.
Taylor-Weiner also discussed how the company’s proprietary ACE Assay™ platform and its rapid development pipeline enable Absci to explore extensive biological space to discover novel variants up to 12 mutations away with best-in-class binding. This approach enabled Absci to create a diverse and novel set of HER2 antibodies with binding affinities that outperform biological baselines using de novo techniques.
Our interview with Absci Chief AI Officer Amaro Taylor-Weiner
Business update and strategic outlook
Zach Jonasson, Absci’s CFO and CBO reported that Absci estimated improvements of greater than 15% in R&D workflow efficiencies over the past year from our AI platform and wet-lab integrations. Absci reduced gross spending even as it increased its number of programs, including internal program development.
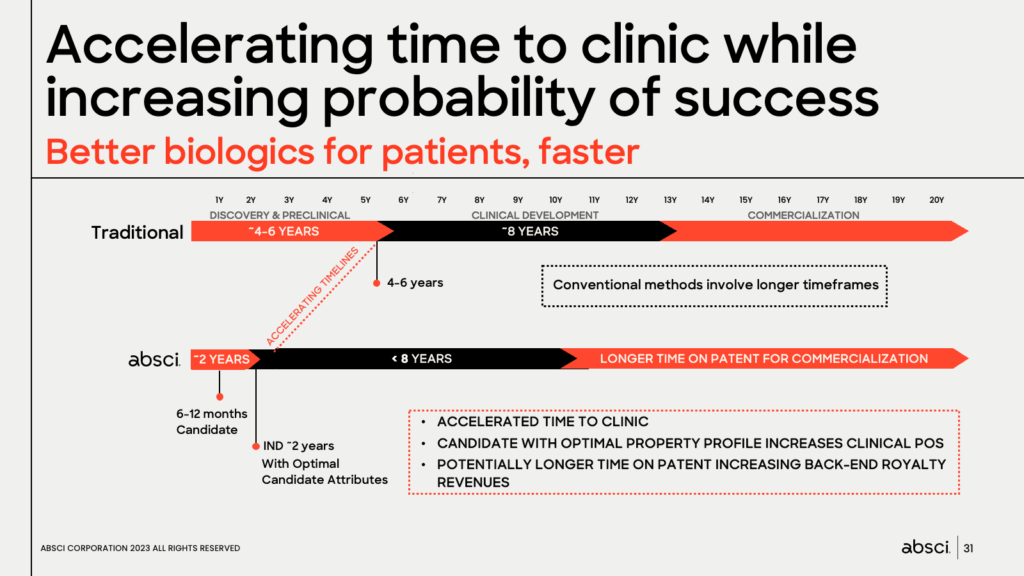
Jonasson also discussed how Absci’s AI drug creation platform has the potential to accelerate the time to the clinic while targeting an increase in the probability of clinical success. He highlighted how the platform’s efficiency leads to cost and speed advantages, with Absci projecting about 2 years to reach IND, compared to the industry’s average of 4-6 years. For drug makers, this means a longer time on patent for commercialization, which could make it economically more feasible to pursue additional treatments for more patients.
Jonasson also shared Absci’s future growth catalysts, including increasing efficiencies in its AI drug creation platform, new partnerships and internal programs, and advances in existing partnerships and programs.