Pediatrician turned pharma innovator Hubert Trübel on using AI to translate R&D into patient-centric solutions.
The path from drug discovery to first-in-human therapies is twisty and time-consuming. Prof. Hubert Trübel has followed a similarly twisty career path, going from academics to medicine to pharma. That experience has contributed to his success in bringing impactful drugs to patients, and it colors how he balances an openness to new technical and professional frontiers with a disciplined eye toward clinical results.
“I have had a long and convoluted career in the pharma sector as well as in the academic world, all driven by chance,” he says. “And so my success factor, I think, is giving chance an opportunity to strike.”
Today, as Chief Medical Officer of AiCuris and a member of Absci’s Scientific Advisory Board, Hubert is less interested in wandering than in finding the most direct path from research to patient impact. He sat down to discuss with us what is top-of-mind for him these days: the shortest path to clinical data, the true meaning of translational success, and AI’s potential for patients with rare diseases.
A convoluted path to drug discovery
Hubert’s interest in translational medicine emerged during his pediatrics residency in Germany when an opportunity came to join a biotech startup. He left his residency and took the plunge, but the company failed just 18 months later. He returned to complete his residency but kept coming up with ideas that couldn’t be pursued in academia.
“I did a fellowship in the US and came back with even more research ideas,” he says. He met some folks at Bayer, and before long he found himself having coffee on Saturday morning at the home of Andreas Busch — then a Bayer executive and Absci’s current Chief Innovation Officer.
He began as a self-described “lab rat” but quickly rose through the ranks as lab head, department head, and experimental medicine lead, all by “following projects along the value chain and translating them into the clinic.” He ultimately led all of Bayer’s clinical research development before moving back to biotech to serve as Chief Medical Officer at AiCuris to help develop anti-infectives.
The shortest path to clinical data
Dr. Trübel brings his translational perspective to Absci’s Scientific Advisory Board, where he feels he’s “in the front seat of antibody development innovation.” His main focus there is on using AI innovations ike designing HCDRs from scratch and multiparametric lead optimization to go from preclinical research to high-value clinical data in the fastest, most direct way possible.
“It’s a long and daunting process at times,” he says, with experimental, manufacturing, and regulatory challenges all along the way. “What made me successful at Bayer, and what I bring to Absci, is thinking about the most efficient way to get to a clinical endpoint to help validate a project. You need the data to show a project is justified to be in your pipeline,” he says.
Hubert loves coming up with ways to validate a target product profile, or TPP. The TPP is the starting point for any new drug. It describes the indications, efficacy, safety, dosing, and delivery of what you’re trying to make. The drug design process is complex and has to be data-driven, he says. But above all else, it must center on patient needs if it hopes to become a blockbuster hit.
“It all starts with an unmet need,” he says. “That’s what drives innovation, whether it’s a drug or anything else you want to bring to market.”
Reverse-engineering the patient experience
In developing the ideal product at the center of the TPP, drugmakers also need to account for other products in the space.
“Let’s talk about PK,” says Hubert. “Imagine there’s another drug on the market for your disease target, and patients take it twice daily. To compete in the market, your drug might need to be once daily.” This, he says, is the basis for what data-driven attributes you need to achieve in your pharmacokinetics — how to keep the active pharmaceutical ingredient in the bloodstream over a period of time, for example. Whatever the PK attribute, the TPP helps you reverse engineer the drug you actually want.
Once you define a drug that meets the needs of patients, physicians, and regulators, “then it’s a matter of going through the hoops,” he says. “You will need to validate different aspects of your target product profile, and today a lot of this validation can be done in silico.”
Translational success in AI drug discovery
Going in silico is key, Hubert says, because the greatest driver of translational success is time saved in achieving a successful clinical data point.
“When you look at drug development today, a lot of time is spent on certain steps in the analysis,” he says. Examples include finding the right antibody-target match or running a dozen assays on millions of compounds. Such processes are extraordinarily expensive, slow, and labor-intensive. And when you finally choose a lead candidate, you then have to optimize it in vitro and in vivo. Hubert believes that a lot of the data needed to validate and optimize candidates is already out there, waiting to be put to use in machine learning models.
“AI will allow us to move faster through these stages,” he says, in a sense enabling drugmakers to jump through more of those hoops with the click of a button. And in terms of peak sales and market exclusivity, Hubert says that the time savings represents enormous value for pharma.
The patient opportunity: Rare diseases and beyond
But the greatest opportunity in AI drug discovery lies in bringing life-changing medicines to patients.
Hubert believes that rare disease communities will be among the first to benefit. Today, drug development for rare diseases depends on the same kinds of animal models and human studies used for other diseases. But this presents a double challenge for rare diseases. First, there are few animal models for rare diseases. And second, it’s often difficult to find enough rare disease patients to conduct a human clinical trial.
Hubert expects that the shift to in silico approaches will ultimately enable AI drugmakers to do more drug development outside of animal models and human studies. He says these technical advances are coming just as regulators are giving drugmakers new leeway in finding treatments for the 6000+ rare diseases impacting humans worldwide.
And rare disease communities will not be the only winners. Whether it’s speeding the discovery process, increasing clinical success, or making more personalized therapies more economically viable, the same AI advances that help rare disease will also advance drug development broadly.
As Hubert puts it: “If AI can shorten the development and resource needs, that’s good for all patients.”
Absci’s AI leader on wet lab integration, partnering with pharma, and his obsession with patient impact.

Amaro Taylor-Weiner, Ph.D., has been scaling solutions in artificial intelligence, healthcare, and computational medicine for more than a decade – long before ChatGPT brought generative AI into global focus. So too, for more than a decade, Absci has been developing its antibody know-how, which it has applied toward its first-in-class ability to design and validate de novo therapeutic antibodies with zero-shot generative AI.
Now, as Absci begins to advance a portfolio of next-generation therapeutic candidates closer to the clinic, Amaro comes aboard as Absci’s Chief AI Officer to help scale the technology that will create better biologics for patients, faster.
“There are many interesting AI opportunities out there, but Absci stands out as the only company with cutting-edge AI, a scalable and integrated wet lab, and a seasoned drug creation team all under one roof,” says Amaro, who has more than 100 highly-cited peer-reviewed publications to his name. “Absci’s ability to generate massive training and validation data in the lab is what excites me and what I believe will ultimately enable AI to bring better antibody therapeutics to the clinic.”
Amaro’s AI-driven genomics discovery has led to new trials and changes in the standard of care. He has also helped develop new AI diagnostics to enable clinical drug development. Absci will benefit from Amaro’s experience in realizing AI-driven innovation as it accelerates toward bringing AI-designed therapeutic candidates to the clinic.
“There’s nothing more powerful than bringing a new therapeutic to market,” and if AI can increase success and speed drugs to market, Amaro says “that’s improving people’s lives in a deep way.”
The path to AI drug discovery
Amaro began his path to AI drug creation after earning his undergraduate degree in cognitive and brain sciences and taking a computational biology role at the Broad Institute. He says working with leading cancer physicians like Dr. Priscilla Brastianos on pediatric brain tumors was the most humbling, inspiring three years of his early career.
“I could see the amazing impact we were having on patients’ lives, and I caught the bug where I was just like, I have to do this – I’m obsessed with this problem,” he says.
To make a bigger impact, Amaro decided to further his studies, earning his Ph.D. in Biomedical Informatics from Harvard. He then spent the next several years growing teams and codebase in biotech. At PathAI, Amaro and his teams developed AI-powered pathology products to advance drug and diagnostic development. As head of data science at Nabla, Amaro developed novel machine-learning methods for computational antibody design. Now, his sights are set on architecting AI for drug creation.
Architecting AI for drug creation
Killer algorithms are only one ingredient of AI success. To bring useful AI-driven innovation to people, Amaro says there’s a lot of “organizational engineering and culture building” that has to happen for AI and wet lab teams to become a well-oiled machine.
“It’s a very different set of problems than writing code,” Amaro says, requiring scientists to make decisions differently, cut through uncertainty, and adopt bold approaches to innovation. He says Absci is doing this, too, as it systematically applies key AI and wet lab technologies to create a portfolio of antibody drug candidates.
“That’s what really drew me to Absci,” he says. “It has this strong base as a wet lab company and a differentiating data advantage over other AI antibody design companies.”
“Amaro is not only a leading AI scientist, but he also brings strong leadership and organizational skills to the table,” says Andreas Busch, Chief Innovation Officer of Absci. “He’s the right guy at the right time for Absci.”
Bringing biotech and AI closer together for pharma
As Amaro gets underway at Absci, one of his goals is to create a more seamless integration between the biotech and AI teams. He points to an eye-opening experience earlier in his career when he was shadowing a colleague in the lab: “Some of the choices that I made in the code meant this guy had to stand there for 16 hours pipetting,” he says.
Amaro says computational groups and wet lab groups tend to work independently with their own jargon and culture. “It’s a new model for the field to have these groups work together tightly and improve each other’s technology through collaboration. We always need to be asking ourselves how we can build each other up.”
With tighter integration comes the opportunity to break down every part of the design loop to speed our iterative development of antibodies using AI, says Amaro. And that’s good when talking to potential partners about what AI design can bring to the pharmaceutical industry.
“To partner with pharma, you need to be specific about how your technology improves their workflows,” says Amaro. “They’re driven by bringing new drugs to market and making accurate decisions about drug assets and won’t be impressed by new technology unless it’s couched in clear value propositions tied to drugs and patients.”
Amaro’s appointment as Chief AI Officer at Absci is a big move towards creating better biologics for patients faster using AI-driven innovation. His experience in realizing AI innovations will be invaluable to Absci as it advances toward bringing therapeutic candidates to the clinic. By scaling solutions in AI, healthcare, and computational medicine, Amaro is poised to help improve patients’ lives in a deep way.
Amir Shanehsazzadeh shares his insider perspective on AI drug discovery and Absci’s de novo breakthrough.

At the Absci Town Hall celebrating the de novo breakthrough, Amir Shanehsazzadeh modestly accepted the mic and joked about not being able to spell ‘antibody’ before coming aboard.
“Of course, I knew what antibodies were,” Amir says, “and that you could use them as a class of therapeutics involved with the immune system. But I really didn’t know any of the details about CDRs, constant regions, variable regions, and so on.”
Amir is an AI scientist at Absci. He uses his computational skills to write the algorithms to design antibody drugs. Amir was raised in a family of doctors — his granddad was a doctor, and his mom and aunt are still doctors. Yet Amir was always drawn more to math than biology.
“My parents kind of wanted me to be a doctor, but I don’t really think that was for me. They gave me an inclination towards life sciences and biology though, which I thought was pretty interesting.”
A career begins
Amir got his first taste of life science research in high school at the Fox Chase Cancer Center in Philadelphia, where he worked as a computational biology intern.
“It turns out, I’m pretty sure I was looking at antibodies,” he says. “At the time, though, I just really didn’t understand the biology. I thought of it from a mathematical perspective.”
While studying math in college, Amir pursued several more internships in the biotech space, including his introduction to using large language models (LLMs) to design proteins. In retrospect, Amir sees these as the early days of the current AI wave.
“Deep learning for proteins is very young — as a field, maybe six or seven years. And I’d say I’ve been doing it for about five of those years. Computational protein design in biology is decades old. But the deep learning stuff is pretty new.” He says deep learning applications in fields like NLP and computer vision really took off in the early 2010s, and then it took a couple of years for people in the life sciences to get familiar with the software and methods. “And now, you know, it’s all the rage in biology.”
In the fall of 2018, Amir applied to intern at a lot of different places, just to get his foot in the door and try things out. He stumbled upon one company with a “kind of basic HTML page that just looked weird.” He wondered if it was even a real company.
The company was very real, in fact. It was D. E. Shaw Research, a company that uses sophisticated computer models to study molecular dynamics, the movements of atoms and molecules. It’s where Amir did his first deep learning work on proteins. It’s also where he met Joshua Meier, Absci’s future Chief AI Officer, who was at Facebook AI Research’s protein team at the time.
Around this time, Amir also applied to an options trading company – he didn’t get the job. Though it might have been a lucrative career, he ultimately found himself inclined toward opportunities where he could see the tangible benefits of his work on people’s lives. “I wanted to make something, whether it was a SaaS product, a consumer product, or a drug.”
Working and studying from home
Amir spent another summer interning at Google Brain, although his plans to split his time between their Mountain View and Cambridge offices were doused by the pandemic. His Google experience was spent at his parents’ home in front of a computer — and then his university went remote. Being stuck at home for his junior year of college gave Amir a lot of time to study, work, and think. He began working full-time at Dyno Therapeutics, a startup using machine learning to design AAV capsids for gene therapy, while also taking a full load of classes.
“I was pretty set on this field, but I began debating whether to work for an industry research lab like Google or Facebook, or go to more of a biotech startup company.” He also began to think about what kind of company could have the biggest impact.
“Whether it’s gene therapy or antibodies, I think it’s hard to argue which of these is better, impact-wise. They’re both trying to cure or treat diseases. Maybe antibodies are 5% more likely to make an impact, or gene therapies might help 1.3 times the number of people. At the end of the day,” Amir says, “if I can help 1000 or 1200 patients with their disease, I’m happy either way.”
As an AI scientist, there was one other factor that impacted Amir’s decision to ultimately join Absci: having faster cycle times in the lab to know how his models are working.
“Being able to get data back faster was really compelling for me. Data is the lifeblood of AI, and in vivo studies typically take a while. For example, it might take nine months to get data back from a non-human primate (NHP) study. I joke that you could have a child in that amount of time. Here, you can get back data from in vitro studies sometimes as fast as a month, usually faster than two months. It’s great.”
Life at Absci
Among Amir’s proudest accomplishments is his role in developing Absci’s de novo antibody design technology.
“Seeing that effort really grow from infancy is what I’m most proud of,” he says. “There’s still a lot of work we have to do, but I think the result we have going from one heavy chain CDR to designing all three heavy chain CDRs successfully is really encouraging. And hopefully, we can extend it to all six CDRs of the antibody.”
Amir says it’s one thing to put it on paper. Now, the next step is getting results to patients.
“How do we start impacting patients’ lives? How do we create a drug with this technology? Because ultimately, that’s the thing that will differentiate us. We have a mandate to create drugs that will improve patients’ lives. That’s what motivates us. And that is where we are all working to make progress.”
Delivering breakthrough biologics to patients requires data from the wet lab and the clinic — a lot of it.
ChatGPT is a remarkable technology that demonstrates the impact that generative AI could have on our lives. It’s also a useful tool for explaining to patients, partners, investors, and even our family and friends what we do at Absci.
In the simplest terms, Absci is building the ChatGPT of biologic drug discovery. But instead of generating text in response to a prompt, Absci generates biologic drug candidates in response to a disease target. Where it typically takes ten years and a billion dollars to bring one new drug to market, generative AI — combined with our ultra-high-throughput screening platform — enables us to create and validate millions of antibody candidates in a matter of weeks, which may shorten the timeline to market. That has the potential to transform the drug industry and help us achieve our mission to bring better biologics to patients faster.
While those two applications of generative AI are completely different, there is one way in which ChatGPT and our AI platform are very much alike: They both depend on massive amounts of training data to be effective. In the case of ChatGPT, that involves reading just about everything ever published digitally and in print. In the case of Absci, to create effective drugs, we need massive amounts of data from protein sequences, structures, and the clinic.
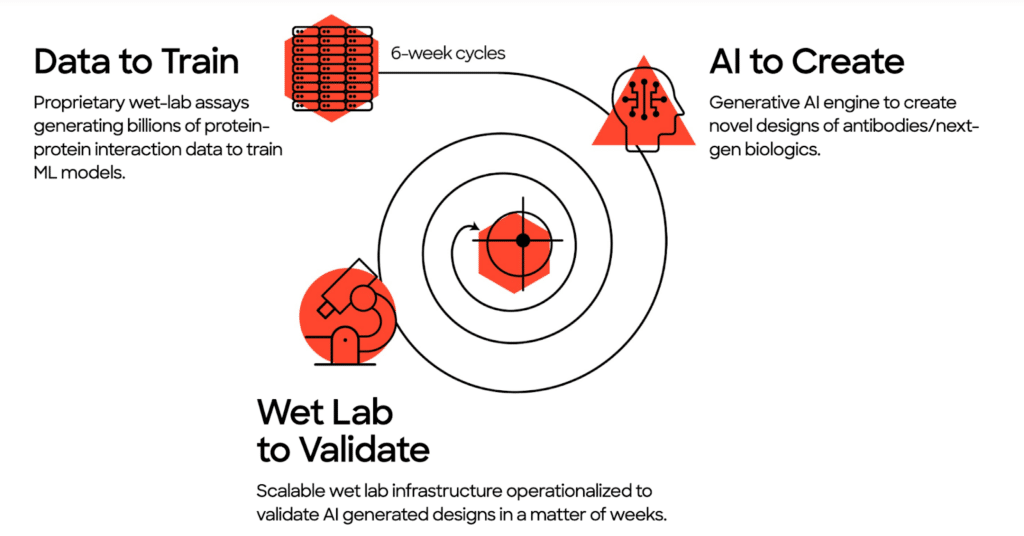
The wet lab data pipeline
Put simply, to have the best AI drug creation platform, you need the best data. To that end, Absci is working to build the world’s premiere data pipeline for AI drug discovery.
There are several public sources of data that are a useful basis from which to begin. These include The Protein Data Bank (protein structures), SAbDab (antibody/antigen complex structures), Observed Antibody Space (antibody sequences), and databases of protein structure like UniProt. AlphaFold, one of the latest and greatest tools for predicting protein structure from sequence, itself uses deep learning AI methods. The use of these and other open-source datasets are table stakes for both traditional discovery and AI drug creation.
Absci builds on public data with our own experimental wet lab data. Our multiplex synthetic biology approach overcomes the limitations of today’s highest-throughput automation labs to create training data at an unprecedented speed, quality, and volume. It has the ability to design and test millions of AI-designed antibodies every week, and that dynamic data is extremely attractive compared to the large but static sample banks of traditional drug discovery.
Absci’s scalable wet lab is a defining feature of AI drug creation and what really sets us apart. Our proprietary wet lab assays generate the massive amounts of biological data needed to enable the predictive power of machine learning. Then, we use that data to design, test, and run experiments in silico (on the computer rather than at a lab bench). This dramatically speeds up the hit finding process to discover unique, generative variants that could never be found using standard methods.
The clinical data pipeline
Besides designing and validating potential therapeutic antibodies for a potential disease target, there’s another aspect to drug discovery: finding and selecting the right target in the first place. As our Chief Innovation Officer and pharma veteran Andreas Busch puts it: “You can produce the best antibody against pretty much any epitope imaginable… But if your target is off, it’s worth nothing.”
That’s where clinical data comes into play. Clinical data is vital to target selection. Absci uses clinical datasets to identify novel antibodies from patients with exceptional immune responses. (For example, antibodies derived from cancer patients with remarkable immune responses have been instrumental in developing targeted cancer therapies.) Then, using our reverse immunology approach, Absci computationally re-assembles antigen-antibody pairs as promising potential starting points for drug development.
But access to clinical data is challenging. That’s because many data sources are owned by companies or institutions that may be reluctant to share their patient-consented research data with others. This can make it difficult for researchers and pharmaceutical companies to access the data they need to develop new drugs, particularly if they do not have the resources to conduct their own clinical trials or studies.
To bring clinical insights to AI drug creation, Absci is partnering with several leading institutions. Together, these data partnerships give Absci a robust clinical data pipeline that enables our AI platform across a wide range of therapeutic areas. They will also help build Absci’s internal therapeutic pipeline:
- St. John’s Cancer Institute (SJCI) is a pioneer in cancer care, research and innovation. In February 2023, SJCI and Absci announced a partnership leveraging SJCI’s leading cancer biospecimen repository and molecular database to uncover breakthrough cancer therapies.
- Aster Insights is a leading oncology data organization with the most advanced lifetime-consented clinicogenomics data to accelerate discovery research. In April 2023, Absci announced it would use Aster’s multi-institutional data set to accelerate the creation of therapeutics for a range of malignancies and patient profiles.
- The University of Oxford’s Kennedy Institute of Rheumatology, a leading biomedical research center for chronic inflammatory and musculoskeletal conditions, announced in May 2023 a partnership with Absci to create breakthrough therapies for immune-mediated diseases. Absci will identify novel antibodies from patients with exceptional immune responses to inflammatory bowel disease (IBD), then reverse engineer promising antibody therapy candidates.
With this incredible clinical data pipeline, we can start to focus our AI drug creation engine on the best possible targets.
A data turning point for pharma
Generative AI is an extremely powerful tool, but it requires massive amounts of high quality data to be effective. That’s true whether you’re using it to craft your emails or creating de novo versions of all three heavy chain CDRs (HCDR123), the antibody regions most critical to target binding. In either case, the old saying applies: “garbage in, garbage out.” Absci is solving the data problem in pharma, first using synthetic biology to generate vast biological data for AI-designed therapeutic candidates, and also with the clinical data to effectively target diseases.
Biotech R&D is at an inflection point. With the emergence of AI drug creation, the industry sees the huge value of data at each step in the drug discovery and development process. Innovators who adopt a systematic data-first approach early on will have a huge advantage in the coming AI transformation toward more efficient and effective drug creation.
More importantly, if we can make this turn as an industry, we are taking a big step toward the holy grail of precision medicine, which rests on the notion of using more and more refined clinical parameters and more and more refined genetic information on patients coming from smaller and smaller patient populations. Ultimately, this is how we will tailor treatments to extremely specific patient populations — perhaps to the individual level.
Data is the key to AI drug creation, both today and tomorrow. We think it’s a huge opportunity, both for our industry and for the patients we aim to serve.
Joining Absci to live the dream of building a successful drug portfolio with maximum patient benefit
In a sense, Christine Lemke’s pharma career has progressed in the same direction she thinks about drug discovery itself: from finish to start.
“I have seen every single part of the pharmaceutical value chain during my career,” Christine says, “starting with the commercial side where I really got into this connection between drugs and drug delivery. I eventually found my way to proteomics, target validation, and even biomarkers — very early in the drug development pathway. So you might say I moved backward through the drug creation process.”
As Senior Vice President of Portfolio and Growth Strategy, Christine will put her understanding of the entire biopharma value chain to full use. She will develop Absci’s internal pipeline of assets and bring those assets and her R&D insights into partnered programs to create better biologics faster.
Beginning with the end in mind
Christine’s philosophy is that taking a patient-centered approach from the beginning is good for customers and essential for drug makers.
“You have to imagine the whole package and how patients will experience your drug, from the way they will take it all the way to reimbursement, and think about that early on,” she says. “If you don’t, it could kill your product.”
The target product profile, or TPP, is one place where drug makers focus on the patient’s point of view. The TPP defines the ideal drug from both a patient and a commercial perspective, considering the relevant patient population and their specific needs and priorities around efficacy, safety, dosing, and administration. It serves as a kind of north star through the complicated drug development process.
Christine says you have to think even more holistically. “The biggest disease burden for a person with chronic lower back pain might be isolation at home due to mobility challenges,” she notes. “Getting out of the house with some help and socializing may be a more important outcome measure than the duration and threshold of pain.”
Making drugs more user-friendly
Christine thinks that technology increasingly offers patients a better experience around the drug itself. She thinks back to her drug delivery experience as one example.
“In chronic disease settings, it is important that drugs come with a good delivery device,” Christine says. “For example, prefilled syringes or pens give customers a new level of convenience and safety. Nowadays, smart pens can even ‘talk to you,’ providing feedback and helping to ensure optimal dosing. This kind of innovation is easy to underestimate. Yes, of course, the foundational innovation is the drug. But from a patient perspective, going to the clinic for an IV injection versus at-home delivery with a smart pen could be the difference between a winning product and a commercial failure.”
“A major challenge for patients in the US as well as for society overall is managing drug costs,” Christine says. “As a biopharmaceutical company, we must think about how to get breakthrough drugs to patients faster and cheaper.” Technologies like zero-shot generative AI have the potential to shorten preclinical development timelines by more than half while also increasing the clinical probability of success. Ultimately, this could decrease drugmakers’ costs and risks, with the savings passed along to patients and society more broadly.
“We are failing if our technology isn’t increasing accessibility to life-changing drugs for the patients everywhere who need them,” she says.
The value that partners bring to drug creation
In designing the patient experience from the beginning, Christine says that big drugmakers are crucial partners in ensuring focus on the patient experience.
“Big biopharmaceutical companies have an intimate understanding of their markets,” she says. “They know the indication space very well, what current and potential future competition looks like, and they know the patient journey and the unmet medical need. In successfully bringing drugs to the market, they have experience and disease-specific networks with patients, caregivers, healthcare professionals, payors, and regulators, enabling them to scale drug development. It is a great opportunity for a company like Absci to collaborate with large biopharmaceutical companies. There are a lot of things that we can learn from their networks.”
Creating an R&D strategy
Combining a manufacturing perspective with patient-centricity, Christine aims to bring a holistic approach to shaping Absci’s R&D strategy. Ultimately, it will guide which market segments Absci taps into and when.
“Absci technology has very broad applicability, but we will not be able to tap into everything at once. We have to stay on a path, understanding what the killer app is today as well as the markets we want to tap into tomorrow,” she says. “We will develop ideas and technologies that we can fully leverage now, and there will be others that may be ready from prime time tomorrow. For some solutions, we may not even be the best owner and they only can get to full potential through partnering or out-licensing.”
Christine says it is tempting for tech companies to burn money trying to realize every potential opportunity. “But it’s just too big of a universe to do everything,” she says. The trick is to generate maximum value through partnerships, licensing, spin-offs, or otherwise.
“We need to be very clear on what we are doing. What capability are we missing, and who is the best partner to achieve it? And what can we give away because it’s not on our path to success? These kinds of questions,” says Christine, “are at the core of an R&D strategy.”
Value writ large
Christine sees a lot of other places where companies like Absci can bring huge value to the world. Addressing health inequalities is chief among them. For example, as shared in Harvard Health, 70% of those affected by chronic pain conditions are women, whereas 80% of pain research is conducted on males. Other studies show that resources are often disproportionately allocated to diseases primarily affecting men.
And when it comes to creating better biologics for patients, Christine already has a strong opinion about where Absci should go.
“I want Absci to be first-in-human for de novo antibodies,” she says. That would not only be a huge victory for patients, she says, but it would also open the floodgates for a world of applications in biologics beyond antibodies.
As Christine embarks on her role at Absci, she’ll be asking lots of questions that take us back to the start.
Pharma veteran Dr. Andreas Busch discusses what drugmakers can reasonably hope to expect from AI drug design in the near-term.
For someone with so much success bringing drugs to market, Andreas Busch comes across as quite the realist.
“We should always remind ourselves what we’re actually affecting versus what we’re doing with the technology,” he says. “You can produce the best antibody against pretty much every epitope imaginable — high affinity, high solubility, high Naturalness, whatever pH you need, you name it — it’s going to be a high-quality antibody. But if your target is off, it’s worth nothing.”
And in case you needed more reality check, Andreas likes to point out that in addition to these scientific challenges there isn’t a blockbuster out there that hasn’t been halted numerous times during its development.
“Drugs get delayed and often put on the shelf for strategic reasons that have nothing to do with their quality. Projects are put on hold because of funding, or perhaps the strategy officer changes. This can obviously play a huge role in the success and speed at which new drugs come to market,” he says.
So, is AI going to fix these problems? No. But Andreas believes AI is going to have a significant impact on biologic drug discovery. Not just in areas that Absci focuses on — antibody design and target discovery — but also in safety, toxicology, and more.
“With present technologies, we can get pretty decent antibodies,” he concedes. “There are many good antibodies on the market already that didn’t use AI. But the value in using generative AI to create de novo antibodies is that we are much faster in optimizing antibodies across multiple parameters.” And that time advantage is huge — especially at the beginning and end of a drug’s life cycle.
In drug discovery, time is more valuable than ever
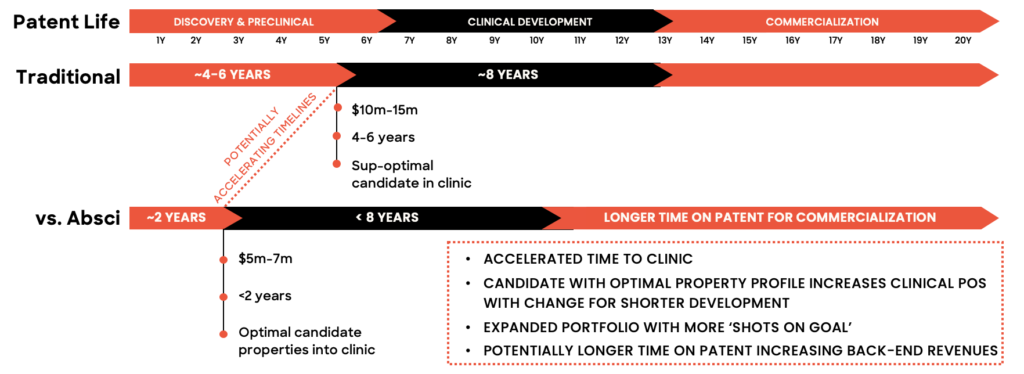
With zero-shot generative AI, Absci thinks it can reduce the time from target identification to clinic by about two years. What’s the consequence of those two years?
“The first is simple to understand,” says Andreas. “If you commercialize your drug two years early, that gives you two more years of sales at the end of your patent life. If you’re talking about a billion-dollar-a-year drug, that could mean $2 billion in revenue at the end of the life cycle. That’s very simple. Generative AI can also provide new opportunities around life cycle management of the drug post-patent cliff.”
The second advantage may be even more important to appreciate, he says, and it pertains to today’s highly competitive drug discovery environment.
“In years past, you often knew whether you had a leadership position on identifying a certain signaling pathway or target,” he says. “Today, whatever target you’re working on, you have to assume that five other companies have also discovered it and are working on it, too. In such cases, a month or two could make the difference between bringing your blockbuster to market and being shut out by a competitor’s patent.”
He adds: “If you have a chance of being two years faster, that puts you in a completely different competitive situation.”
If mice could talk
In addition to the time advantage, Andreas believes that AI can just do a better job of optimizing antibodies across multiple parameters than conventional approaches.
“If you do a mouse campaign to identify an antibody, you can’t tell a mouse, ‘We don’t want just any antibody from you – it has to be highly soluble, have the right binding, the right pH dependence, and so on.’” Andreas says you can get high quality antibodies, but it’s going to take years to get all the characteristics you want.
“With predictive AI approaches, we can optimize antibodies to have many specific parameters all at once.”
“And if our predictions around what we call a Naturalness score are correct, then we can predict things like immunogenicity, which is of huge importance to pharma – making sure you don’t form autoantibodies or any immunogenic reactions. That’s a huge value to the drugmaker.”
IP opportunities through AI
Andreas believes that because generative AI can also provide numerous highly diverse sequences that bind the same epitope, a drugmaker’s IP estate has the potential to be meaningfully expanded.
“The zero-shot AI approach is a method that specifically removes any training data for the specific targets of interest,” Andreas explains. “So we’re teaching it the general principles of good antibody design, but we’re not biasing it toward known antibody sequences in existing libraries.”
Andreas says that this approach generates designs that are quite diverse compared to known antibodies, giving drug makers greater opportunities with regard to patent estate strategy.
In our recent bioRxiv manuscript, we showed how we can use zero-shot generative AI to design HCDR3 regions for trastuzumab with extreme edit distances and higher affinities.
”When we do talk to partners about patent opportunities, they understand the potential value really well.”
Creating drugs at the speed of AI
The field of AI drug discovery is moving fast and changing the way drugmakers think about technology partnerships, addressable markets, and the drug discovery process itself. It’s moving so fast, in fact, that Andreas believes we’re shifting from a paradigm of drug discovery to one of drug creation.
“First serving on Absci’s Board, and now as Chief Innovation Officer, I’m more convinced than ever that Absci is dismantling the paradigm of drug discovery with generative AI,” says Andreas, “allowing us to achieve our vision to go from discovering or finding drugs within existing libraries to creating drugs in silico on a computer.”
In our August 2022 manuscript published in bioRxiv, we explored using our machine learning models to simultaneously optimize binding affinity and naturalness scores of the therapeutic antibody trastuzumab. Read it to learn more about how we are leveraging generative AI and scalable biological data to lead the protein drug creation revolution:
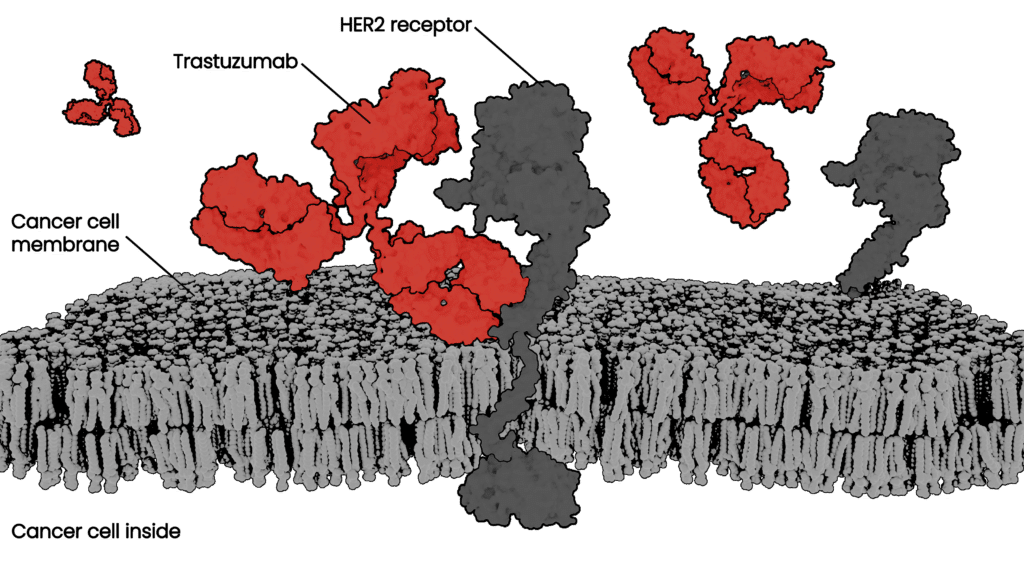
The therapeutic antibody field has experienced explosive growth as biologics have become the predominant class of new drugs developed by major players in the pharmaceutical industry. Antibodies are natural drugs produced by our immune system and very promising therapeutics, especially in cancer and immune diseases. Their power comes from natural selectivity – they can bind to specific target proteins, which can be engineered to block or change biological processes underlying disease. Also, a properly designed therapeutic antibody will be recognized by our body as part of natural defenses without invoking an immune response or unwanted side effects.
Over the past several years, biologics progressed toward being the top best-selling drugs – seven out of ten blockbusters in 2022 – creating an extremely competitive therapeutic niche. Identifying novel methods to optimize these drug candidates’ safety, efficacy, and manufacturability in the shortest time possible has become more critical than ever.
How To Develop A Therapeutic Antibody?
Therapeutic antibodies traditionally undergo five significant sequential stages leading up to preclinical development. First, the drug’s target is investigated for its relevance in the disease based on primary research. Then the antibody is generated by immunizing an animal and growing its immune cells that produce the antibodies. These antibodies are initially tested in the lab, and those that look most promising pass on to the next step, initial lead selection. These lead drug candidates undergo lead optimization, a critical stage in which the antibodies are honed for biological activity and properties that will impact the drug’s efficacy, safety, and developability. Finally, the optimized lead candidates go through preclinical enabling to make them suitable for animal testing.
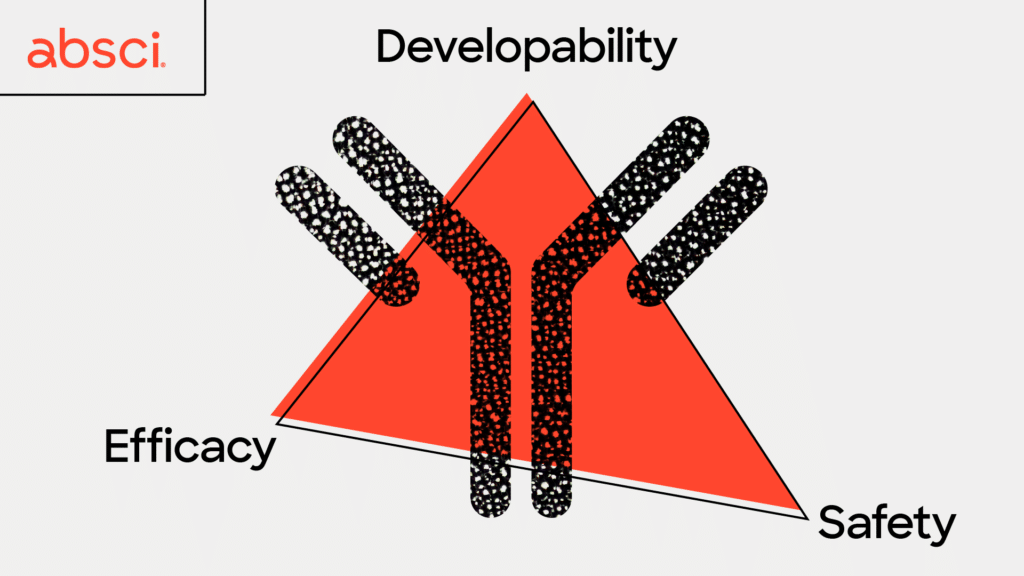
Lead optimization is one of the most vital steps in antibody development. It plays an essential role in maximizing an antibody’s likelihood of clinical success and ultimately becoming an approved drug. Unfortunately, this step is also extremely expensive, time-consuming, and plagued with a high rate of failure. One of the biggest challenges is that lead optimization must simultaneously assure efficacy, safety, and developability. It is a very common case that perfecting one parameter ruins another – a process that has often been described as playing a game of ‘whack-a-mole’ where improving one property may lead to unintended effects on other properties and so on in a perpetual cycle that may never yield an ideal candidate.
Two of the most important properties to optimize – or moles to simultaneously knock down – before preclinical studies are efficacy and safety. These are indicators that an antibody will have a strong therapeutic effect and be less likely to have severe side effects. In order to have high efficacy a good therapeutic antibody candidate will ideally only attach, or bind, to very specific cells in your body, such as cancer cells. This allows the cancer cell to be destroyed by the immune system while leaving healthy surrounding tissue intact. The antibody binding must be not only specific, but also very strong and long-lasting to give immune cells time to kill the cancer cells. The strength of this binding is defined as affinity, and is a key metric drug developers seek to optimize for therapeutic antibodies.
Another indicator of whether a therapeutic antibody will produce severe side effects is immunogenicity – or whether the antibody is likely to trigger an unwanted immune response. Immunogenicity in particular, can be a tricky parameter to gauge in a single experiment due to the complexity of the immune system and all the different cells and proteins a therapeutic antibody may encounter on the way to its target. However, it has been hypothesized that the more similar an antibody is to those occurring in nature, the less likely they will be to create an immune response. With this in mind, we have been developing a new metric called “naturalness”. We believe that therapeutic antibodies with high naturalness scores will be less likely to trigger an immune response in patients, leading to fewer adverse effects and better outcomes.
Stronger, Better, Faster: Machine Learning To The Rescue
To design a promising lead drug candidate for further development, you need to find antibodies with several different properties, all falling within a small range. It’s like searching for a needle in a haystack, except the haystack is larger than the known universe, and the needle is smaller than a speck of sand. Using traditional discovery methods, this can be a long, expensive process with a little probability of success. At Absci we’ve designed our generative AI models to significantly speed up and improve the lead optimization process compared to traditional methods that rely solely on wet lab screening. Using our proprietary wet lab assays we generate the massive amounts of biological data needed to enable the predictive power of machine learning. Using computers to design, test, and run experiments in silico (on the computer rather than at a lab bench) we can dramatically speed up the lead optimization process and discover unique, generative variants that could never be found using standard methods.
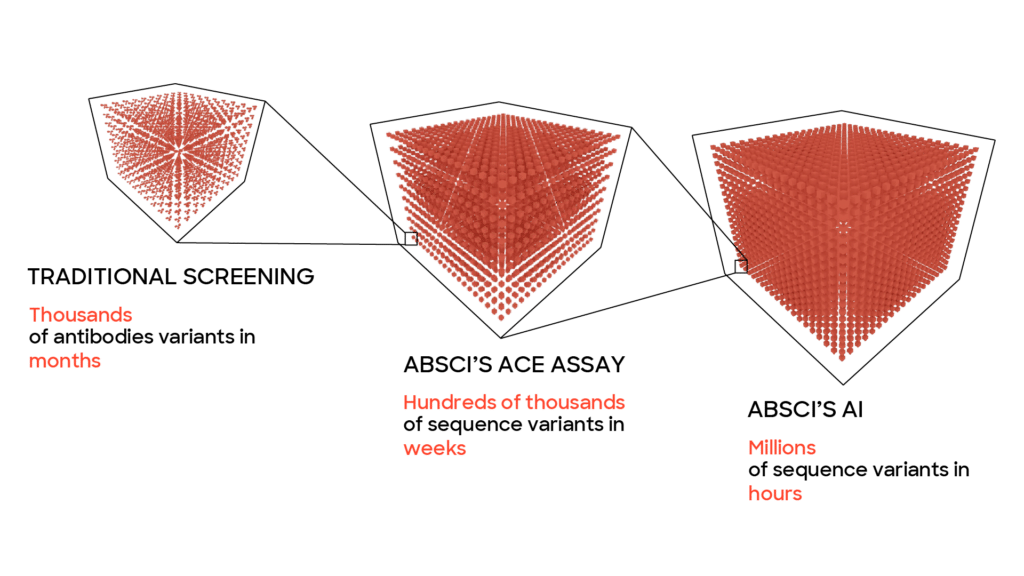
One reason this method is able to work faster than traditional methods is that machine learning models allow us to test a much larger number of potential drugs – far more than would be possible or practical to explore exclusively in a wet lab due to time and money considerations. With our platform, we can quickly probe a wide variety of vastly different potential drugs and then hone in on the most promising ones extremely quickly. To do what would take years using traditional methods can be done in months or weeks. For the lead optimization phase of a project this enables the computer to play our metaphorical whack-a-mole game at a breakneck pace until it finds an antibody where all the parameters have been optimized and the therapeutic antibody can continue to preclinical studies.
Let’s Work Together to Bring Therapeutic Antibodies to Patients Faster!
We work with our partners on target and antibody discovery, AI-assisted antibody optimization, and site-specific payload conjugation via nsAA incorporation. Connect with us today to see how we can help you create better biologics.