ABS-101
Absci has applied our Integrated Drug Creation™platform to go from de novo design to a differentiated TL1A candidate in 14 months.
Absci has applied our Integrated Drug Creation™ platform to go from target to promising candidates in 14 months.
Overview
Potential best-in-class TL1A mAb for IBD
- Extended half-life
- Monomeric & trimeric binding
14 months from AI design to IND-enabling studies
Currently in IND-enabling studies
Targeting TL1A
TL1A is a pro-inflammatory cytokine of the TNF-receptor superfamily that binds to Death Receptor 3 (DR3), initiating signaling cascades leading to pro-inflammatory and pro-fibrotic responses. TL1A is implicated in multiple inflammatory and fibrotic diseases beyond IBD including: rheumatoid arthritis, atopic dermatitis, lupus erythematosus, psoriasis, intestinal fibrosis, pulmonary fibrosis, and liver fibrosis.


De novo design and AI lead optimization produced diverse TL1A candidates
Our de novo model performed global and local epitope landscaping, exhaustively sampling interface contacts across the antigen to refine potency and MoA.
With a known target, epitopes, and MoA, our generative AI engine created sets of antibodies with particular scaffold sequences, leading to millions of diverse leads. We then used AI to refine and optimize leads across clinical parameters like affinity and potency, identifying several candidates.
Our wet lab functionally validated leads in a matter of weeks, identifying several with novel epitope interactions.
Selected epitope enabled high affinity binding to both the TL1A human monomer/trimer
Estimated performance of clinical competitor reagent generated for in-house comparison.
We used BLI values for comparing monomer and trimer binding and not as absolute values due to sensitivity limits for the instrument at high affinity.
Selected epitope enabled high affinity binding to both the TL1A human monomer/trimer
Affinity by biolayer interferometry (BLI)

Target to promising leads in just over 12 months
3 lead candidates were identified of which we progressed the most promising one into IND enabling activities.

Preclinical profile


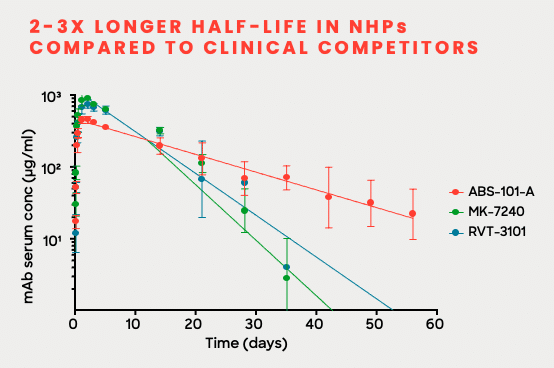
Our de novo design and AI lead optimization models created a potentially best-in-class TL1A candidate with high affinity, superior potency, and an extended half-life that allows for longer dosing intervals.
IND-enabling studies

IND-enabling studies ongoing for ABS-101
Optimization parameters
Sub-Q formulation
Favorable PK with long half-life
Safety
High Bioavailability


